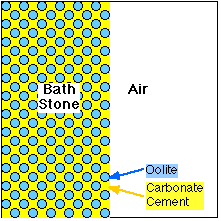
 Oolites bound firmly with Calcium Carbonate cement |
|||
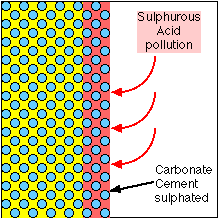 Calcium Carbonate attacked by Sulphur to become Calcium Sulphate |
|||
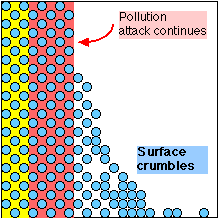 Oolites loosened as Sulphate expands and dissolves - Stonework destroyed |
|||
CHEMICAL EROSION
Bath Stone is known as 'Oolitic Limestone'. It is made up of hard round particles, "Oolites", bound together with a naturally occuring Calcium Carbonate "cement".
When exposed to an atmosphere which contains Sulphur, a chemical reaction occurs which replaces the Carbonate in the cement with Sulphate. The Sulphate is a softer, bulkier material which swells the surface of the stone, pushing the Oolites apart and allowing them to be eroded. It is also soluble in water, so gradually dissolves away, leaving the Oolites without any binding cement.
 Oolites bound firmly with Calcium Carbonate cement |
|||
 Calcium Carbonate attacked by Sulphur to become Calcium Sulphate |
|||
 Oolites loosened as Sulphate expands and dissolves - Stonework destroyed |
|||
This damage becomes obvious as the surface of the stone is seen to crumble or flake away; but, by the time this is noticed, the chemical damage will have extended much deeper. The stone on many buildings in Bath is already chemically 'programmed' to self-destruct to a depth of up to 1 cm.
Until the 1950s and 60s, coal was the main fuel for domestic heating in Bath, many factories also used coal to fire boilers for steam power and process heating. The resulting fumes were rich in Sulphur Dioxide which was converted by rain into dilute Sulphurous Acid (acid rain), this was the chief cause of stonework damage in Bath.
When the Clean Air Act brought an end to coal burning, an extensive programme of stonework cleaning was undertaken in Bath. It was hope that, with reduced atmospheric Sulphur levels, the buildings would continue to stay clean. In fact, they were found to be deteriorating at an even faster rate, even though the remaining atmospheric Sulphur (which was now due to traffic pollution) was at a much lower level than in the previous coal-burning decades.
Research work by Professor Geoff Allen at Bristol University discovered that the particular mixture of Sulphur and Nitrogen compounds in traffic fumes could cause the sulphating reaction to speed up and, in some cases, the rate of attack was increased to 24 times faster than that of the Sulphur alone.
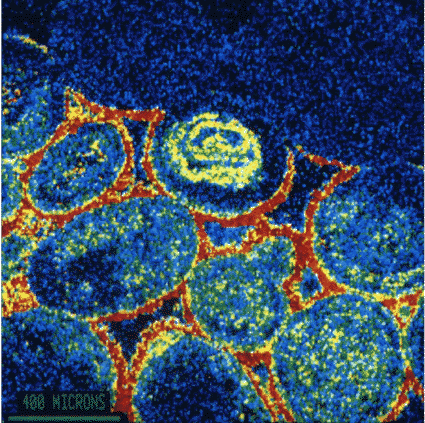 |
This picture is in 'false colour' where each colour represents a different chemical element. It is a section of the surface of a piece of Bath Stone which has been attacked by atmospheric pollution The round Oolites are about 500 microns across and show-up in blue with yellow speckles. The binding cement of naturally occuring Carbonates was between the Oolites. Because has been converted to Sulphate, it shows up in red which is the 'false colour' of Sulphur in this picture.
Picture: Professor Geoff Allen, |
Back to Effects of Pollution
on Bath
Back to Environmental pages
More about Pollution in a Heritage City?
More
about Bath's Traffic Problems?